New Method for Insulin Delivery Using Stem Cells
There are many types of diabetes mellitus including juvenile-onset diabetes (type 1), adult-onset diabetes (type 2), and diabetes during pregnancy (gestational diabetes). The underlying mechanism of all diabetes is dysfunctional or non-existent excretion of insulin from the islet cells of the pancreas or insulin insensitivity at the glucagon receptor of the liver and muscle. According to the CDC, type 1 diabetes affects approximately 1.6 million Americans, including 187,000 children and adolescents. Type 1 diabetes is thought to be an autoimmune disorder where slow destruction of the pancreatic beta cells of the islets of Langerhans leads to the cessation of insulin secretion and usually begins within early adolescents. This form of diabetes requires daily insulin injections to keep blood glucose levels at an acceptable physiological range.
Insulin is a small protein peptide that unfortunately cannot be formulated into an oral delivery device due to the harsh environment of the stomach that would denature the protein as well as the insulin that survives not being able to cross the intestinal epithelium due to its size thus a subcutaneous injection is currently the only method to bypass these physiological barriers.
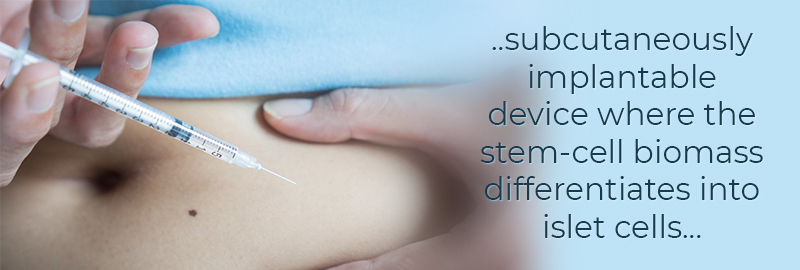
Recently, researchers at ViaCyte Inc. have designed a new and novel method for insulin delivery. Using stem cells, they have constructed a subcutaneously implantable device where the stem-cell biomass differentiates into islet cells, releasing insulin in a physiologically regulated fashion. Seventeen participants were enrolled in a phase1/2 clinical trial to test their VC-02 device. The device is made of a multi-layered ‘sandwich’ that includes a semi-permeable membrane with engineered portals and an external polyester mesh providing rigidity to the device. Inside the device are engrafted pluripotent stem cell-derived pancreatic endoderm cells (PEC-01). The PEC-01 cells are derived from ethically sourced human embryos and is a cell population that contains pancreatic progenitor and immature endocrine cells. This device allows for the direct vascularization of the biomass enabling the cells to sense fluctuations of blood glucose levels; thus, excreting the proper amount of insulin.
The biomarker used to monitor the device’s success and efficacy is C-peptide produced by the pancreas, is a byproduct during insulin production, and has a significantly longer serum half-life; therefore, making it easier to monitor. The 17 participants enrolled had an age range of 22-57 years, and all had a diagnosis of type 1 diabetes. Of the participants, 63% were observed to have successful engraftment and insulin excretion. Out of the 17 participants, 6 (35.3%) showed positive C-peptide as early as 6-month post-implantation. This device is not without its limitations, 27.9% of the enrolled participants experienced adverse side effects related to the surgical implantation or explant procedures, and 33.7% had a side effect to the immunosuppressant therapy needed to avoid acute/chronic rejection of the device. This research is the first step of many needed to provide a natural memetic alternative for insulin secretion. It may in the future reduce or eliminate the need for daily injections and monitoring of blood glucose.
Visit us at Axxiem.com